-
Photoexcitation and Relaxation Dynamics of Catecholato–Iron(III) Spin-Crossover Complexes
C. Enachescu, A. Hauser, J.-J. Girerd and M.-L. Boillot
ChemPhysChem, 7 (2006), p1127-1135


DOI:10.1002/cphc.200500671 | unige:3649 | Abstract | Article HTML | Article PDF
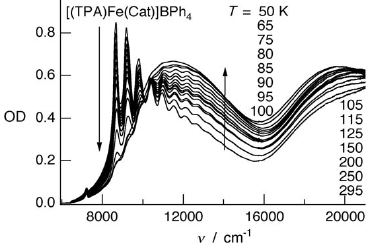
The photophysical properties of the ferric catecholate spin-crossover compounds [(TPA)Fe(R-Cat)]X (TPA=tris(2-pyridylmethyl)amine; X=PF6-, BPh4-; R-Cat=catecholate dianion substituted by R=NO2, Cl, or H) are investigated in the solid state. The catecholate-to-iron(III) charge-transfer bands are sensitive both to the spin state of the metal ion and the charge-transfer interactions associated with the different catecholate substituents. Vibronic progressions are identified in the near-infrared (NIR) absorption of the low-spin species. Evidence for a low-temperature photoexcitation process is provided. The relaxation dynamics between 10 and 100 K indicate a pure tunneling process below ≈40 K, and a thermally activated region at higher temperatures. The relaxation rate constants in the tunneling regime at low temperature, kHL(T→0), vary in the range from 0.58 to 8.84 s-1. These values are in qualitative agreement with the inverse energy-gap law and with structural parameters. A comparison with ferrous spin-crossover complexes shows that the high-spin to low-spin relaxation is generally faster for ferric complexes, owing to the smaller bond length changes for the latter. However, in the present case the corresponding rate constants are smaller than expected based on the single configurational coordinate model. This is attributed to the combined influence of the electronic configuration and the molecular geometry.